Abstract

Iron-induced Heart Failure (HF) still remains the main cause of mortality in patients with thalassemia major (TM), although the introduction of the T2* Cardiovascular Magnetic Resonance (CMR) for the non-invasive assessment of Myocardial Iron Overload (MIO) led to a significant increase in survival rate. However, the pathophysiology of heart failure in TM can be multifactorial. Thanks to its multiparametric potential, CMR represents a unique tool for characterization of myocardial involvement. It is the gold standard for the quantification of biventricular size and function by cine images and for the detection of replacement fibrosis by late gadolinium enhancement (LGE) technique.
The aim of this multicenter study was to evaluate the prognostic value of multiparametric CMR in predicting death for HF.
We considered 1398 white TM patients who performed a baseline CMR exam within the Myocardial Iron Overload in Thalassemia (MIOT) network. As per inclusion criteria, no patient had a history of HF. Mean age was 30.8±8.9 years and 725 (51.9%) patients were females.
During a mean follow-up of 4.83±2.05 years, 49.1% of the patients changed at least once the chelation regimen (i.e.: either switched to a different type of chelator or underwent dose/frequency modification); these patients were more likely to have a global heart T2* value<20ms than patients who maintained the same regimen (33.2% vs 19.7%; P<0.0001)
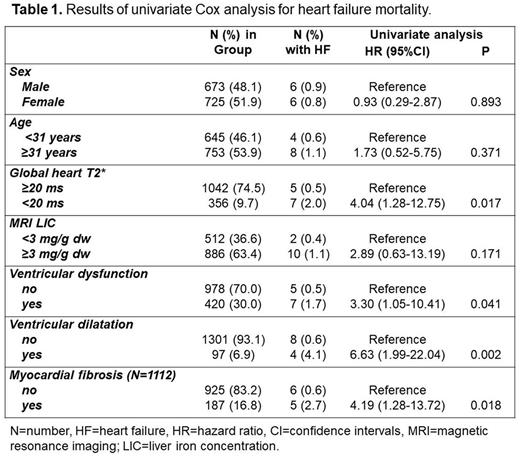
Twelve (1.0%) patients died from heart failure. Table 1 shows the results, presented as hazard ratio (HR) with 95% confidence intervals (CI), of the univariate Cox Regression analysis, performed to test the association between the considered prognostic variables and the outcome. No association was detected between age or gender and HF mortality. Significant MIO (global heart T2*<20 ms), ventricular dysfunction, ventricular dilation, and replacement myocardial fibrosis were identified as significant univariate prognosticators. Due to the low number of deaths for HF, it was not possible to perform a multivariate model. However, based on the presence of the four CMR predictors of HF death, patients were divided into three subgroups: four markers (7 patients; one HF death-14.3%), one to four markers (617 patients, 9 HF deaths-1.5%), and none of the four markers (488 patients; one HF death-0.2%). Patients having all four markers had a significantly higher risk of dying for HF than patients without markers (HR=89.93; 95%CI=5.62-1439.46; P=0.001) or with one to three CMR markers (HR=12.69; 95%CI=1.60-100.36; P=0.016).
In our homogeneous white and well-treated Italian/Mediterranean population, we detected a low incidence of deaths for HF, since the T2* report guided the patient-specific adjustment of the chelation regimen. As expected, MIO was a significant predictor of HF death but also other CMR parameters, namely ventricular dilatation, ventricular dysfunction, and replacement myocardial fibrosis emerged as unfavorable prognosis determinant. Importantly, when the four CMR indices were evaluated in combination, they fine-tuned the prognostic stratification of TM patients. Hence, the findings of the present study promote the exploitation of the full potential of CMR, including LGE, for a better risk stratification of TM patients.
Disclosures
Maggio:Vertex: Membership on an entity's Board of Directors or advisory committees; Celgene Corp (Bristol Meyers Squibb): Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Cademartiri:Bayer: Other: no profit support; Chiesi Farmaceutici S.p.A: Other: no profit support.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal